




Listen as expert oncologists discuss the most important things to know about platinum-resistant ovarian cancer.
annual diagnosed cases in the US1
most common
gynecologic malignancy2
of patients pass away from
late-stage disease3
Ovarian cancer is diagnosed in stages III to IV in approximately 75% of cases4
Nonspecific symptoms common in ovarian cancer can be misattributed to other conditions or health issues.5




Defining platinum resistance
Ovarian cancer with a treatment-free interval of <6 months since the last
line of treatment with platinum chemotherapy7
~85% of patients experience recurrence and become platinum resistant7
Women diagnosed with ovarian cancer typically undergo surgery and platinum-based chemotherapy during frontline treatment.8
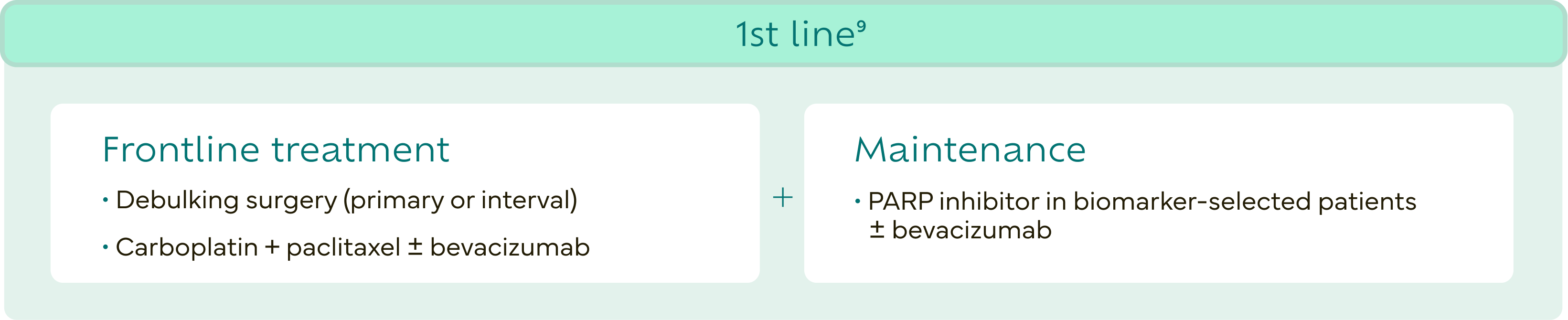
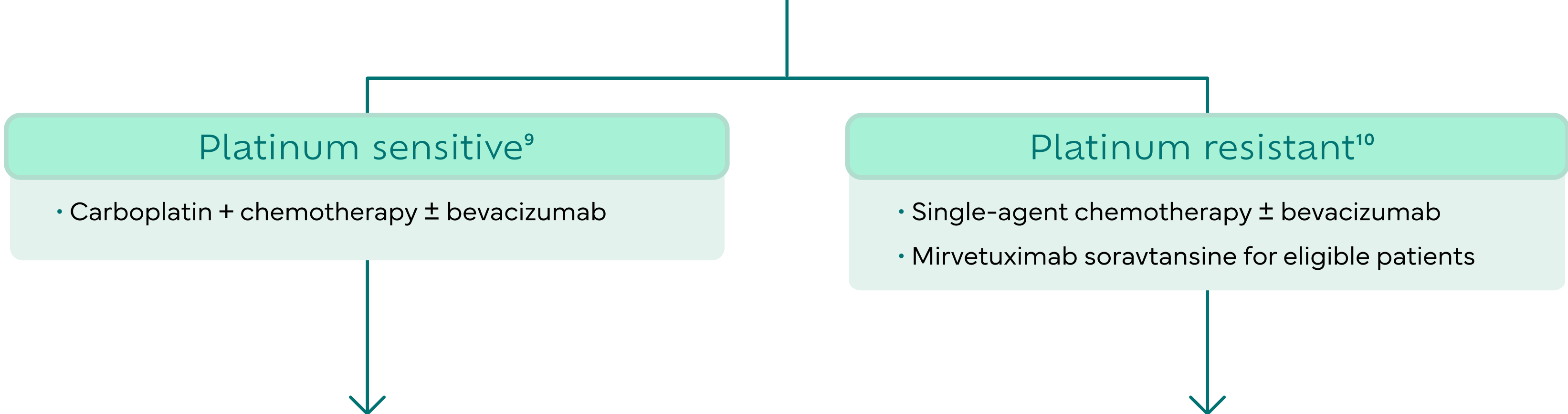

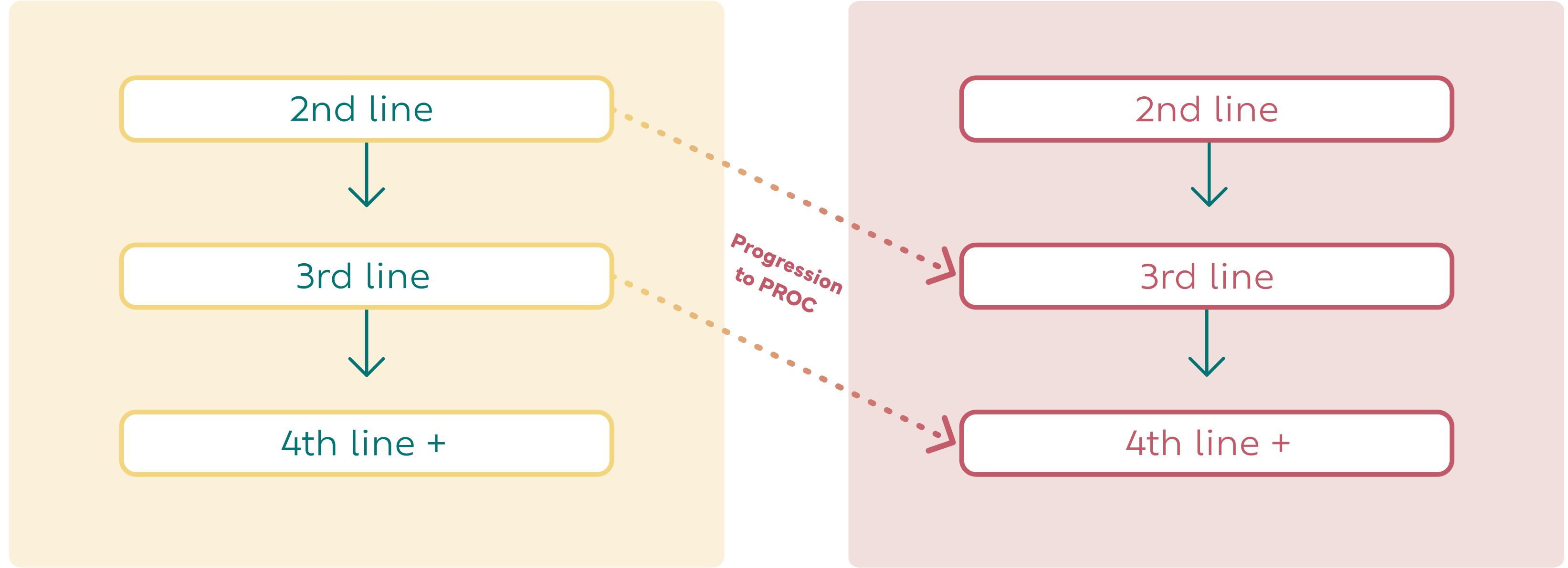
The realities of PROC are challenging
Despite recent advancements, results with standard-of-care treatment leave women with a challenging journey.11

become resistant to platinum chemotherapy7
median progression-free survival with current options12

median overall survival with current options12

are eligible for FDA-approved biomarker therapy11
Management is focused on balancing tolerability, quality of life, and
outcomes for each individual patient11,12
There has been 1 therapy approved by the FDA for platinum-resistant ovarian cancer in the last decade. Treatment outcomes are typically poor, with disease progression in approximately 3 to 4 months following single-agent chemotherapy.11-13
Platinum-resistant ovarian cancer treatment options include single-agent chemotherapy ± bevacizumab, biomarker-targeted therapies, and investigational therapies. However, the side effects and modest efficacy of current therapies can make treatment decisions challenging.8,11,14
New studies are elucidating previously unknown resistance mechanisms in ovarian cancer. Ongoing research and innovative pipeline candidates may lead to new treatments and improve outcomes in the management of platinum-resistant ovarian cancer.15
PARP, poly (ADP-ribose) polymerase inhibitor; PROC, platinum-resistant ovarian cancer.
1. National Cancer Institute. Cancer Stat Facts: Ovarian Cancer. Surveillance, Epidemiology, and End Results Program. 2025. Accessed June 18, 2025. https://seer.cancer.gov/statfacts/html/ovary.html 2. Arter ZL, Desmond D, Berenberg JL, Killeen JL, Bunch K, Merritt MA. Epithelial ovarian cancer survival by race and ethnicity in an equal-access healthcare population. Br J Cancer. 2024;130(1):108-113. doi:10.1038/s41416-023-02471-z 3. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280-304. doi:10.3322/caac.21559 4. Ghirardi V, Fagotti A, Ansaloni L, et al. Diagnostic and therapeutic pathway of advanced ovarian cancer with peritoneal metastases. Cancers (Basel). 2023;15(2):407. doi:10.3390/cancers15020407 5. Chan JK, Tian C, Kesterson JP, et al. Symptoms of women with high-risk, early-stage ovarian cancer. Obstet Gynecol. 2022;139(2):157-162. doi:10.1097/AOG.0000000000004642 6. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151-156. doi:10.1016/j.soncn.2019.02.001 7. St Laurent J, Liu JF. Treatment approaches for platinum-resistant ovarian cancer. J Clin Oncol. 2024;42(2):127-133. doi:10.1200/JCO.23.01771 8. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Ovarian Cancer. 2024. July 19, 2024. Accessed December 11, 2024. https://www.nccn.org/patients/guidelines/content/PDF/
ovarian-patient.pdf 9. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian cancer, version 2.2020. J Natl Compr Canc Netw. 2021;19(2):191-226. doi:10.6004/jnccn.2021.0007 10. Lui JF. Symptoms of women with high-risk early-stage ovarian cancer. Abstract presented at: NCCN 2023 Annual Conference; May 2023; Oxford, UK. 11. Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41(13):2436-2445. doi:10.1200/JCO.22.01900 12. Richardson DL, Eskander RN, O’Malley DM. Advances in ovarian cancer care and unmet treatment needs for patients with platinum resistance: a narrative review. JAMA Oncol. 2023;9(6):851-859. doi:10.1001/jamaoncol.2023.0197 13. FDA approves mirvetuximab soravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer. FDA. March 22, 2024. Accessed December 11, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant-epithelial-ovarian 14. Osman MA, Elkady MS, Nasr KE. Weekly paclitaxel versus three-weekly paclitaxel in recurrent platinum-resistant epithelial ovarian and peritoneal cancers: a phase III study. Clin Med Insights Oncol. 2016;10:35-41. doi:10.4137/CMO.S38204 15. Colombo N, Van Gorp T, Matulonis UA, et al. J Clin Oncol. 2023;41(30):4779-4789.